Solved
Consider the Figure Below Which Shows G° for a Chemical Process Plotted Against Absolute Temperature
Multiple Choice
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.From this plot,it is reasonable to conclude that: 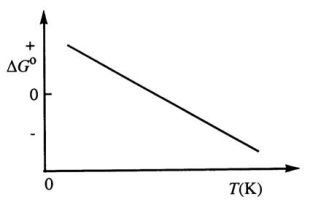
A) ( H° > 0, S° > 0)
B) ( H° > 0, S° < 0)
C) 9 H° < 0, S° > 0)
D) ( H° < 0, S° < 0)
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: For a given reaction, a change in
Q10: State the second and third laws of
Q12: For a chemical reaction to be spontaneous
Q43: Which of the following is true for
Q58: In some spontaneous processes, the entropy of
Q66: Which of the following values is
Q70: The temperature at which the following process
Q71: Use the given data at 298
Q72: Nitric oxide reacts with chlorine to
Q102: When a sky diver free-falls through the