Multiple Choice
Diatomic N2 can react with diatomic H2 to form ammonia (NH3) .The balanced chemical equation is: 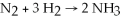
If 6 moles of H2 totally reacted with more than enough N2,how many moles of ammonia would be expected to form?
A) 2 moles
B) 3 moles
C) 4 moles
D) 6 moles
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Suppose a computer manufacturer can build circuit
Q27: Global warming is due to the greenhouse
Q46: The percent yield is calculated by dividing
Q50: What is the limiting reactant for the
Q54: Given the recipe: 2 cups flour +
Q63: Given the recipe: 2 cups flour +
Q75: How many moles of water are needed
Q77: What is the percent yield of CuS
Q83: How many grams of calcium phosphate are
Q88: Many metals react with halogens to give