Multiple Choice
What is the percent yield of CuS for the following reaction given that you start with 
Of Na2S and 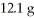
CuSO4? The actual amount of CuS produced was 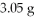
.
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) 16.1%
B) 42.1%
C) 18.93%
D) 7.25%
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: When viewing a chemical equation,the limiting reactant
Q12: Suppose a computer manufacturer can build circuit
Q27: Global warming is due to the greenhouse
Q54: Given the recipe: 2 cups flour +
Q63: Given the recipe: 2 cups flour +
Q73: Which is the limiting reactant in the
Q75: How many moles of water are needed
Q79: Diatomic N<sub>2</sub> can react with diatomic H<sub>2</sub>
Q83: How many grams of calcium phosphate are
Q88: Many metals react with halogens to give