Multiple Choice
A 24.0 g sample of nitrogen gas reacts with an excess of hydrogen gas to give an actual yield of 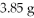
NH3.What is the percent yield for this reaction?
Reaction: N2(g) + 3 H2(g) → 2 NH3(g)
A) 86.8%
B) 73.6%
C) 26.4%
D) 13.2%
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q7: For the following reaction you have 8
Q41: What is the limiting reactant for the
Q44: Consider a reaction of chemicals depicted as
Q49: Hydrochloric acid reacts with barium hydroxide according
Q49: Consider the reaction: 2 Al + 3Br<sub>2</sub>
Q69: How many grams of sodium metal are
Q77: Before determining conversion factors,it is necessary to
Q89: Consider the following generic chemical equation: 2W
Q97: Given that 4 NH<sub>3</sub> + 5 O<sub>2</sub>
Q99: Stoichiometry is a chemist's version of following