Multiple Choice
Hydrochloric acid reacts with barium hydroxide according to the equation: 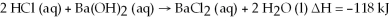
Calculate the heat (in kJ) associated with the complete reaction of 18.2 grams of HCl (aq) .
A) -58.9
B) +58.9
C) -29.5
D) -236
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: For the following reaction you have 8
Q44: Consider a reaction of chemicals depicted as
Q45: A 24.0 g sample of nitrogen gas
Q49: Consider the reaction: 2 Al + 3Br<sub>2</sub>
Q89: Consider the following generic chemical equation: 2W
Q93: The average global temperature depends on all
Q98: How many moles of chlorine gas are
Q99: Stoichiometry is a chemist's version of following
Q108: In order to determine the limiting reactant
Q110: How many moles of aluminum are needed