Multiple Choice
What is the theoretical yield in grams of CuS for the following reaction given that you start with 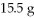
Of Na2S and 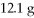
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) 0.0758
B) 0.198
C) 18.93
D) 7.25
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: A chemist wishes to perform the following
Q23: Given the balanced equation CO<sub>2</sub> + Si
Q41: The limiting reactant is not necessarily the
Q46: The percent yield is calculated by dividing
Q57: How many moles of lithium nitrate are
Q59: The conversion factor between mass and moles
Q62: What is the theoretical yield of waffles
Q64: How many moles of aluminum oxide are
Q79: What is the limiting reactant for the
Q92: Starting with 156 g <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="Starting