Multiple Choice
Starting with 156 g 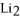
O and 33.3 g 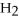
O,decide which reactant is present in limiting quantities.
Given: 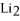
O + 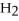
O → 2 LiOH
A) lithium oxide
B) water
C) lithium hydroxide
D) insufficient data
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: A chemist wishes to perform the following
Q23: Given the balanced equation CO<sub>2</sub> + Si
Q57: How many moles of lithium nitrate are
Q62: What is the theoretical yield of waffles
Q64: How many moles of aluminum oxide are
Q79: What is the limiting reactant for the
Q89: What is the theoretical yield in grams
Q96: A balanced chemical equation used to prepare
Q100: Thermal energy flows into the reaction and
Q102: Given the chemical equation: 2 Ca +