Multiple Choice
If the theoretical yield of the reaction below corresponds to 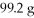
And the actual yield was 60.9 g,calculate the percent yield.
Given: 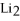
O + 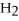
O → 2 LiOH
A) 16.0 %
B) 71.8 %
C) 38.0 %
D) 61.4 %
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Consider the following reaction: 2 Mg +
Q22: The limiting reactant is the reactant that
Q26: The conversion factor for moles of carbon
Q42: Given the recipe: 2 cups flour +
Q45: The limiting reactant is the product that
Q53: How many moles of water are made
Q73: The percent yield can never be greater
Q98: What is the excess reactant for the
Q100: Thermal energy flows into the reaction and
Q101: How many grams of water are needed