Multiple Choice
What is the excess reactant for the reaction below given that you start with 10.0 grams of Al and 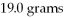
Of O2?
Reaction: 4Al + 3O2 → 2Al2O3
A) Al
B) O2
C) Al2O3
D) both Al and O2
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: The conversion factor for moles of carbon
Q45: The limiting reactant is the product that
Q53: How many moles of water are made
Q62: What is the theoretical yield of waffles
Q79: What is the limiting reactant for the
Q96: A balanced chemical equation used to prepare
Q100: Thermal energy flows into the reaction and
Q101: How many grams of water are needed
Q102: Given the chemical equation: 2 Ca +
Q102: If the theoretical yield of the reaction