Multiple Choice
How much energy does it take to melt a 16.87 g ice cube? 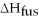
= 6.02 kJ/mol
A) 102 kJ
B) 108 kJ
C) 936 J
D) 5.64 kJ
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Ionic solids tend to have higher melting
Q18: The change of a substance from a
Q23: NaCl is which type of solid?<br>A)molecular solid<br>B)ionic
Q30: A 250 gram sample of water at
Q31: Which compound in liquid form will have
Q40: Which intermolecular force is present in all
Q53: Assuming that the molecules carbon monoxide (CO)and
Q56: The tendency of a liquid to minimize
Q79: The strength of the surface tension is
Q110: The boiling point is the temperature at