Multiple Choice
A 250 gram sample of water at the boiling point had 45.0 kJ of heat added.How many grams of water were vaporized? Heat of vaporization for water is 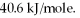
A) 1.11
B) 20.0
C) 0.902
D) 16.2
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q18: The change of a substance from a
Q25: How many kJ of heat are needed
Q26: Which intermolecular forces are found in <img
Q31: Which compound in liquid form will have
Q32: Which substance below has dipole-dipole forces?<br>A)CH<sub>4</sub><br>B)CO<sub>2</sub><br>C)F<sub>2</sub><br>D)H<sub>2</sub>S<br>E)none of
Q33: How much energy does it take to
Q53: Assuming that the molecules carbon monoxide (CO)and
Q56: The tendency of a liquid to minimize
Q79: The strength of the surface tension is
Q110: The boiling point is the temperature at