Multiple Choice
How many kJ of heat are needed to completely melt 1.70 moles of 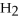
O,given that the water is at its melting point? The heat of fusion for water is 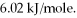
A) 30.6
B) 10.2
C) 3.54
D) 63.7
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Ice can float in a glass of
Q22: Dry ice (solid C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="Dry
Q31: Which noble gas has the highest boiling
Q49: Which of the following statements is false?<br>A)Evaporation
Q51: Increasing the temperature increases the vaporization rate
Q71: Viscosity increases with increased intermolecular force because
Q76: The reason for many of the unique
Q85: Evaporation is:<br>A)increased by increasing temperature.<br>B)an endothermic process.<br>C)the
Q90: The measure of the resistance to the
Q91: Water has unusual physical properties which enable