Multiple Choice
Dry ice (solid C 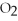
) is which type of solid?
A) molecular solid
B) ionic solid
C) covalent atomic solid
D) nonbonding atomic solid
E) metallic atomic solid
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: How many kJ of heat are needed
Q25: How many kJ of heat are needed
Q26: Which intermolecular forces are found in <img
Q31: Which noble gas has the highest boiling
Q32: Which substance below has dipole-dipole forces?<br>A)CH<sub>4</sub><br>B)CO<sub>2</sub><br>C)F<sub>2</sub><br>D)H<sub>2</sub>S<br>E)none of
Q71: Viscosity increases with increased intermolecular force because
Q76: The reason for many of the unique
Q82: A situation where two opposite processes are
Q90: The measure of the resistance to the
Q106: What happens as you start to add