Multiple Choice
Consider the following image, showing on the atomic level, the reaction occurring when a Zn wire is placed in a copper(II) nitrate solution. 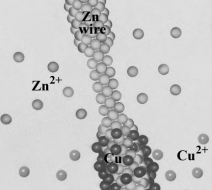 Which of the following correctly describes the image?
Which of the following correctly describes the image?
A) Zinc is being oxidized.
B) Copper is being reduced.
C) Zinc is the reducing agent.
D) All of the above are correct descriptions.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the batteries listed below is
Q2: Which of the following are paired correctly?<br>A)
Q3: Consider the following reaction.<br>3Fe<sup>2+</sup>(aq) + 2Al(s)
Q4: Consider the following reaction.<br>3Fe<sup>2+</sup>(aq) + 2Al(s)
Q6: Which one of the following will not
Q7: Which of the following is the function
Q8: The reaction(s) found in primary batteries are
Q9: When a zinc strip is placed in
Q10: Which of the following equations does
Q11: Free radicals are particles that<br>A) have an