Consider the Following Reaction 3Fe(s) + 2Al3+(aq)
This Reaction Takes Place in the Electrochemical
Short Answer
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below. 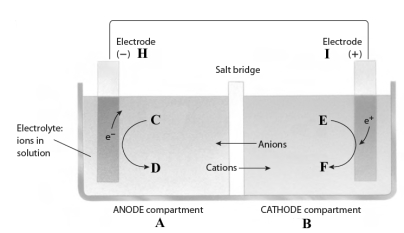
Complete the following questions using the letters shown in the image.
-The oxidizing agent is found in compartment ______.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the batteries listed below is
Q2: Which of the following are paired correctly?<br>A)
Q3: Consider the following reaction.<br>3Fe<sup>2+</sup>(aq) + 2Al(s)
Q5: Consider the following image, showing on the
Q6: Which one of the following will not
Q7: Which of the following is the function
Q8: The reaction(s) found in primary batteries are
Q9: When a zinc strip is placed in
Q10: Which of the following equations does
Q11: Free radicals are particles that<br>A) have an