Multiple Choice
Calculate the 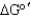 of a redox reaction of pyruvate and hydrogen under standard conditions using the table below.
of a redox reaction of pyruvate and hydrogen under standard conditions using the table below. 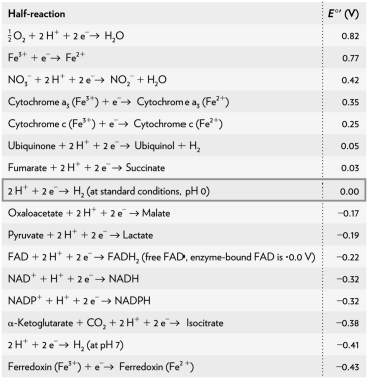
A) 18.33 kJ/mol
B) 36.66 kJ/mol
C) 54.99 kJ/mol
D) 73.32 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q45: Identify the strongest reductant in the table
Q46: Which reactions in the citrate cycle produce
Q47: Which reaction in the citrate cycle produces
Q48: Briefly describe the eight reactions from the
Q49: Coenzyme A does not readily cross the
Q51: Explain the effect a high concentration of
Q52: The anaplerotic reaction catalyzed by pyruvate carboxylase
Q53: In the citrate cycle,a high concentration of
Q54: Why is the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6697/.jpg" alt="Why is
Q55: Identify the coenzyme that provides a reactive