Multiple Choice
Why is the 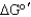 of the condensation of oxaloacetate and acetyl-CoA highly favorable?
of the condensation of oxaloacetate and acetyl-CoA highly favorable?
A) The addition of inorganic phosphate provides energy, resulting in the highly favorable nature of this reaction.
B) Allosteric regulations of the reaction cause a release of energy, making the reaction thermodynamically favorable.
C) The iron-sulfur cluster in the enzyme reduces the activation energy of the reaction, resulting in the favorable 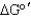
D) The hydrolysis of the thioester bond in citryl-CoA results in the highly exergonic reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q49: Coenzyme A does not readily cross the
Q50: Calculate the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6697/.jpg" alt="Calculate the
Q51: Explain the effect a high concentration of
Q52: The anaplerotic reaction catalyzed by pyruvate carboxylase
Q53: In the citrate cycle,a high concentration of
Q55: Identify the coenzyme that provides a reactive
Q56: Which citrate cycle intermediate is siphoned off
Q57: Which reaction in the citrate cycle produces
Q58: Explain why riboflavin is critical to the
Q59: The primary function of NAD<sup>+</sup> in the