Multiple Choice
Sodium chlorate is used as an oxidizer in the manufacture of dyes, explosives, and matches. Calculate the mass of solute needed to prepare 1.575 L of 0.00250 M NaClO3 ( 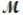 = 106.45 g/mol) .
= 106.45 g/mol) .
A) 419 g
B) 169 g
C) 0.419 g
D) 0.169 g
E) 0.00394 g
Correct Answer:

Verified
Correct Answer:
Verified
Q13: How many moles of H<sup>+</sup>(aq) ions are
Q73: Aluminum metal dissolved in hydrochloric acid as
Q74: Magnesium carbonate, MgCO<sub>3</sub>, is insoluble. Identify the
Q76: Select the classification for the following reaction.
Q79: Select the best statement relating to the
Q80: Complete and balance the equation for the
Q81: Aqueous potassium iodate (KIO<sub>3</sub>) and potassium iodide
Q82: Calculate the molarity of a 23.55-mL solution
Q93: Which of the following solutions will be
Q107: All acid-base reactions produce a salt and