Multiple Choice
Calculate the molarity of a 23.55-mL solution which contains 28.24 mg of sodium sulfate (used in dyeing and printing textiles, 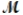 = 139.04 g/mol) .
= 139.04 g/mol) .
A) 8.625 M
B) 1.199 M
C) 0.8339 M
D) 0.2031 M
E) 0.008625 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: How many moles of H<sup>+</sup>(aq) ions are
Q78: Sodium chlorate is used as an oxidizer
Q79: Select the best statement relating to the
Q80: Complete and balance the equation for the
Q81: Aqueous potassium iodate (KIO<sub>3</sub>) and potassium iodide
Q84: A 0.1873 g sample of a pure,
Q85: Copper(II) sulfide, CuS, is used in the
Q86: Calculate the oxidation number of the chlorine
Q87: Aqueous potassium iodate (KIO<sub>3</sub>) and potassium iodide
Q107: All acid-base reactions produce a salt and