Multiple Choice
How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 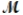 = 84.02 g/mol) ? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
= 84.02 g/mol) ? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
A) 638 mL
B) 572 mL
C) 536 mL
D) 276 mL
E) 175 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Select the precipitate that forms when aqueous
Q22: Which of the following is most soluble
Q30: Sulfuric acid (H<sub>2</sub>SO<sub>4</sub>) reacts with potassium hydroxide
Q32: Select the net ionic equation for the
Q33: In each of the following cases, write
Q34: Select the precipitate that forms when the
Q39: In a redox reaction, the oxidizing agent
Q60: How many moles of ions are released
Q74: Which one of the following substances is
Q90: Which of the following is a weak