Multiple Choice
Select the correct set of quantum numbers (n, l, ml, ms) for the highest energy electron in the ground state of potassium, K.
A) 4, 1, -1, 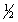
B) 4, 1, 0, 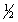
C) 4, 0, 1, 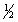
D) 4, 0, 0, 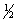
E) 4, 1, 1, 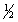
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Select the most basic compound from the
Q3: Elements in which the outermost electron has
Q8: Hund's rule is used to predict the
Q32: Define what is meant by electron affinity,
Q34: An atom of element number 33 (As)
Q36: Which one of the following equations correctly
Q38: First ionization energies of neutral atoms may
Q38: Consider the set of isoelectronic atoms and
Q74: Which of the following elements has the
Q76: The most acidic oxides are formed from