Multiple Choice
An atom of element number 33 (As) is in its ground electronic state. Which one of the following sets quantum numbers could not apply to any of its electrons?
A) n = 2 l = 1 ml = -1 ms = + 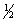
B) n = 3 l = 0 ml = 0 ms = - 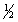
C) n = 3 l = 2 ml = -2 ms = - 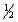
D) n = 4 l = 0 ml = 0 ms = - 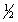
E) n = 4 l = 2 ml = 1 ms = + 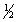
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Select the most basic compound from the
Q29: In Mendeleev's version of the periodic table,
Q29: Elements with _ first ionization energies and
Q31: State Hund's rule, and show how it
Q32: Define what is meant by electron affinity,
Q36: Which one of the following equations correctly
Q37: Select the correct set of quantum numbers
Q38: First ionization energies of neutral atoms may
Q38: Consider the set of isoelectronic atoms and
Q76: The most acidic oxides are formed from