Multiple Choice
Benzaldehyde ( 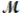 = 106.1 g/mol) , also known as oil of almonds, is used in the manufacture of dyes and perfumes and in flavorings. What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m, freezing point of pure ethanol = -117.3°C.
= 106.1 g/mol) , also known as oil of almonds, is used in the manufacture of dyes and perfumes and in flavorings. What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m, freezing point of pure ethanol = -117.3°C.
A) -117.5°C
B) -118.7°C
C) -119.0°C
D) -120.6°C
E) < -121°C
Correct Answer:

Verified
Correct Answer:
Verified
Q23: The Tyndall effect<br>A)is observed in concentrated solutions.<br>B)is
Q52: Colligative properties depend on<br>A)the chemical properties of
Q76: Calculate the freezing point of a solution
Q77: From the following list of aqueous solutions
Q77: Octane and nonane are liquids which are
Q78: Children under the age of six with
Q79: Isoamyl salicylate ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg" alt="Isoamyl salicylate
Q83: List five important intermolecular forces that operate
Q85: A saturated solution of carbon dioxide in
Q89: The chemist, Anna Lytic, must prepare 1.00