Multiple Choice
Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 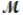 = 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
= 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
A) -1.7°C
B) -0.9°C
C) 0.0°C
D) 0.9°C
E) 1.7°C
Correct Answer:

Verified
Correct Answer:
Verified
Q66: A solution of ethanol (C<sub>2</sub>H<sub>6</sub>O) in water
Q71: Potassium hydrogen phosphate is used in the
Q72: Predict the ideal van't Hoff factor (i)
Q74: A 0.137 m solution of sodium ferrocyanide
Q75: Which of the following pairs of ions
Q77: Octane and nonane are liquids which are
Q77: From the following list of aqueous solutions
Q78: Children under the age of six with
Q79: Isoamyl salicylate ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg" alt="Isoamyl salicylate
Q81: Benzaldehyde ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg" alt="Benzaldehyde (