Multiple Choice
What is the mass-action expression, Qp, for the following reaction? SbF5(g) + 4Cl2(g)  SbCl3(g) + 5ClF(g)
SbCl3(g) + 5ClF(g)
A) 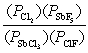
B) 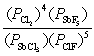
C) 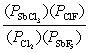
D) 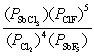
E) None of the above is the correct mass-action expression.
Correct Answer:

Verified
Correct Answer:
Verified
Q38: The equilibrium constant, K<sub>p</sub>, has a value
Q39: The reaction system POCl<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg" alt="The
Q40: The Haber process for ammonia synthesis is
Q41: Ammonia is synthesized in the Haber process:<br>N<sub>2</sub>(g)
Q44: The equilibrium constant K<sub>c</sub> for the reaction
Q45: Nitric oxide and bromine were allowed to
Q47: Sodium hydrogen carbonate decomposes above 110°C to
Q48: Carbon monoxide and chlorine combine in an
Q85: If Q > K, more products need
Q88: In a chemical reaction, if the starting