Multiple Choice
Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 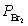 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
A) 7.45 × 10-3
B) 0.109
C) 9.18
D) 91.8
E) 134
Correct Answer:

Verified
Correct Answer:
Verified
Q40: The Haber process for ammonia synthesis is
Q41: Ammonia is synthesized in the Haber process:<br>N<sub>2</sub>(g)
Q43: What is the mass-action expression, Q<sub>p</sub>, for
Q44: The equilibrium constant K<sub>c</sub> for the reaction
Q47: Sodium hydrogen carbonate decomposes above 110°C to
Q48: Carbon monoxide and chlorine combine in an
Q49: Write the mass-action expression, Q<sub>c</sub>, for the
Q50: At high temperatures, carbon reacts with O<sub>2</sub>
Q85: If Q > K, more products need
Q88: In a chemical reaction, if the starting