Multiple Choice
Write the mass-action expression, Qc , for the following chemical reaction. Zn(s) + 2Ag+(aq)  Zn2+(aq) + 2Ag(s)
Zn2+(aq) + 2Ag(s)
A) 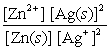
B) 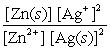
C) 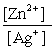
D) 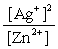
E) 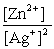
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Hydrogen iodide, HI, is formed in an
Q29: SO<sub>2</sub> reacts with O<sub>2</sub> to produce SO<sub>3</sub>.
Q31: A good catalyst for a reaction will
Q49: Write the mass-action expression, Q<sub>c</sub>, for the
Q50: At high temperatures, carbon reacts with O<sub>2</sub>
Q51: The reaction system CS<sub>2</sub>(g) + 4H<sub>2</sub>(g) <img
Q52: The following reaction is at equilibrium at
Q53: Stearic acid, nature's most common fatty acid,
Q56: The following reaction, in CCl<sub>4</sub> solvent, has
Q58: The equilibrium constant for reaction (1) below