Multiple Choice
Write the mass-action expression, Qc, for the following chemical reaction equation. 2C6H6(g) + 15O2(g)  12CO2(g) + 6H2O(g)
12CO2(g) + 6H2O(g)
A) 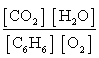
B) 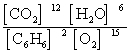
C) 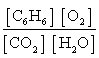
D) 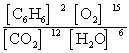
E) 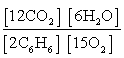
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: The equilibrium constant K<sub>c</sub> for the reaction
Q45: Nitric oxide and bromine were allowed to
Q47: Sodium hydrogen carbonate decomposes above 110°C to
Q48: Carbon monoxide and chlorine combine in an
Q50: At high temperatures, carbon reacts with O<sub>2</sub>
Q51: The reaction system CS<sub>2</sub>(g) + 4H<sub>2</sub>(g) <img
Q52: The following reaction is at equilibrium at
Q53: Stearic acid, nature's most common fatty acid,
Q54: Write the mass-action expression, Q<sub>c </sub>, for
Q88: In a chemical reaction, if the starting