Multiple Choice
Write the mass-action expression, Qc , for the following chemical reaction. Fe3+(aq) + 3OH-(aq)  Fe(OH) 3(s)
Fe(OH) 3(s)
A) 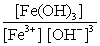
B) 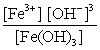
C) 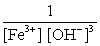
D) 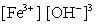
E) 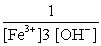
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Increasing the initial amount of the limiting
Q10: At 850°C, the equilibrium constant K<sub>p</sub> for
Q12: Consider the following two equilibria and their
Q13: Methanol can be synthesized by combining carbon
Q14: Hydrogen bromide will dissociate into hydrogen and
Q14: The two equilibrium constants for the same
Q18: The following reaction is at equilibrium in
Q53: When a reaction system reaches equilibrium, the
Q61: For some gas-phase reactions, K<sub>p</sub> = K<sub>c</sub>.
Q78: If all the reactants and products in