Multiple Choice
Consider the following two equilibria and their respective equilibrium constants: (1) NO(g) + 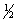 O2(g)
O2(g)  NO2(g)
NO2(g)
(2) 2NO2(g)  2NO(g) + O2(g)
2NO(g) + O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
A) K2 = 2/K1
B) K2 = (1/K1) 2
C) K2 = -K1/2
D) K2 = 1/(2K1)
E) K2 = 1/(2K1) 2
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Unless <font face="symbol"></font>H°<sub>rxn</sub> = 0, a change
Q8: Consider the equilibrium<br>H<sub>2</sub>(g) + Br<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg"
Q9: About half of the sodium carbonate produced
Q9: Increasing the initial amount of the limiting
Q10: At 850°C, the equilibrium constant K<sub>p</sub> for
Q13: Methanol can be synthesized by combining carbon
Q14: Hydrogen bromide will dissociate into hydrogen and
Q15: Write the mass-action expression, Q<sub>c </sub>, for
Q53: When a reaction system reaches equilibrium, the
Q61: For some gas-phase reactions, K<sub>p</sub> = K<sub>c</sub>.