Multiple Choice
Calculate S° for the reaction 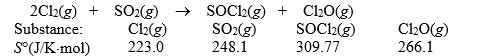
A) -118.2 J/K
B) -104.8 J/K
C) 104.8 J/K
D) 118.2 J/K
E) 1270.0 J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: For a given reaction, a change in
Q9: Which one of the following changes of
Q10: State the second and third laws of
Q11: A chemical reaction has <font face="symbol"></font>H° =
Q12: For a chemical reaction to be spontaneous
Q14: Calculate <font face="symbol"></font>S° for the reaction <img
Q15: Calculate <font face="symbol"></font>G°<sup> </sup>for the reaction of
Q16: For a chemical reaction to be spontaneous
Q17: You are given pure samples of ethane,
Q18: For each of the following pairs, predict