Essay
For each of the following pairs, predict which (A or B) will have the greater entropy, and in one sentence indicate your reasoning. 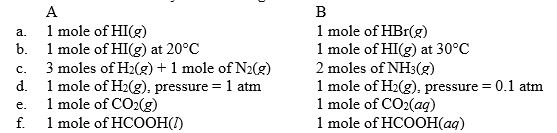
Correct Answer:

Verified
a. A has greater entropy. HI and HBr are...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q13: Calculate <font face="symbol"></font>S° for the reaction <img
Q14: Calculate <font face="symbol"></font>S° for the reaction <img
Q15: Calculate <font face="symbol"></font>G°<sup> </sup>for the reaction of
Q16: For a chemical reaction to be spontaneous
Q17: You are given pure samples of ethane,
Q19: A certain process has <font face="symbol"></font>H° >
Q20: The formation constant for the reaction <br><img
Q21: You are given pure samples of pentane,
Q23: The temperature at which the following process
Q33: Under a given set of conditions, all