Multiple Choice
Consider the figure which shows G° for a chemical process plotted against absolute temperature. 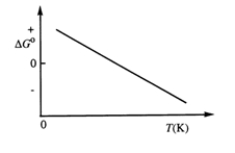 Which one of the following is an incorrect conclusion, based on the information in the diagram?
Which one of the following is an incorrect conclusion, based on the information in the diagram?
A) .H° > 0
B) .S° > 0
C) The reaction is spontaneous at high temperatures.
D) .S° increases with temperature while H° remains constant.
E) There exists a certain temperature at which H° = TS°.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: a. Explain what is meant by a
Q3: Elemental boron can be formed by reaction
Q4: Which relationship best describes <font face="symbol"></font>S° for
Q5: Compare one mole of ice with one
Q6: Iron(III) oxide can be reduced by carbon
Q8: For a given reaction, a change in
Q9: Which one of the following changes of
Q10: State the second and third laws of
Q11: A chemical reaction has <font face="symbol"></font>H° =
Q12: For a chemical reaction to be spontaneous