Multiple Choice
Iron(III) oxide can be reduced by carbon monoxide. 
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature. 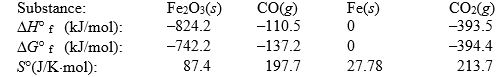
A) 7.0 × 10-6
B) 1.3 × 10-3
C) 2.2 × 104
D) 1.4 × 105
E) > 2.0 × 105
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Given: H<sub>2</sub>O(l) <font face="symbol"></font> H<sub>2</sub>O(g) <font face="symbol"></font>H°
Q2: a. Explain what is meant by a
Q3: Elemental boron can be formed by reaction
Q4: Which relationship best describes <font face="symbol"></font>S° for
Q5: Compare one mole of ice with one
Q7: Consider the figure which shows <font face="symbol"></font>G°
Q8: For a given reaction, a change in
Q9: Which one of the following changes of
Q10: State the second and third laws of
Q11: A chemical reaction has <font face="symbol"></font>H° =