Multiple Choice
Which one of the following equations represents the formation reaction of CH3OH(l) ?
A) C(g) + 2H2(g) + 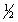 O2 (g) CH3OH(l)
O2 (g) CH3OH(l)
B) C(g) + 4H(g) + O(g) CH3OH(l)
C) C(graphite) + 4H(g) + O(g) CH3OH(l)
D) C(diamond) + 4H(g) + O(g) CH3OH(l)
E) C(graphite) + 2H2(g) + 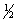 O2(g) CH3OH(l)
O2(g) CH3OH(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q3: What is the final temperature when 20.0
Q18: A 275-g sample of nickel at 100.0°C
Q35: A system absorbs 21.6 kJ of heat
Q51: a. State Hess's Law.<br>b. Use the <font
Q52: Calcium hydroxide, which reacts with carbon dioxide
Q52: Your favorite candy bar, Gummy Beakers, contains
Q55: a. Write a balanced equation for the
Q56: Benzene is a starting material in the
Q57: The standard heat (enthalpy) of formation of
Q59: Which one of the following is not