Essay
a. State Hess's Law.
b. Use the H° data given below to calculate H° for the reaction: 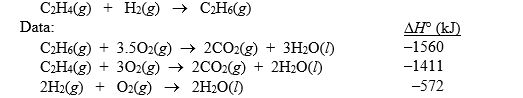
Correct Answer:

Verified
a. The enthalpy change for an ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
a. The enthalpy change for an ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q18: A 275-g sample of nickel at 100.0°C
Q46: Use Hess's Law to calculate the enthalpy
Q52: Your favorite candy bar, Gummy Beakers, contains
Q52: Calcium hydroxide, which reacts with carbon dioxide
Q54: Which one of the following equations represents
Q55: a. Write a balanced equation for the
Q56: Benzene is a starting material in the
Q57: The standard heat (enthalpy) of formation of
Q59: The standard state of a substance in
Q67: Natural gas, or methane, is an important