Multiple Choice
What is the hybridization for each of the indicated atoms in the following compound? 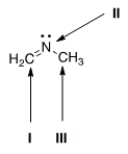
A) I = sp2; II = sp2; III = sp2.
B) I = sp2; II = sp3; III = sp3.
C) I = sp; II = sp2; III = sp3.
D) I = sp2; II = sp2; III = sp3.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Which of the following statements about electronegativity
Q5: Which of the following statements about constitutional
Q7: Avobenzone is an active ingredient in some
Q8: How many constitutional isomers are there for
Q9: What is the hybridization of the carbon
Q9: Follow the curved arrows to draw the
Q10: Which of the following molecules has nonpolar
Q32: What is the ground-state electronic configuration of
Q56: What is the approximate value of the
Q62: How many different isomers are there for