Multiple Choice
Refer to the Bohr model of methane shown below. 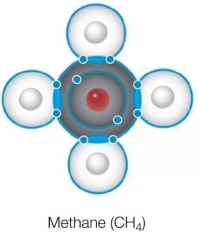 Which statement about this structure is true?
Which statement about this structure is true?
A) All bonds are ionic bonds.
B) All bonds are hydrogen bonds.
C) All bonds contain paired electrons from carbon.
D) All bonds contain paired electrons from hydrogen.
E) All bonds contain paired electrons shared between carbon and hydrogen.
Correct Answer:

Verified
Correct Answer:
Verified
Q156: The mass number of an atom is
Q157: The covalent bonds in methane, CH<sub>4</sub>, are
Q158: Refer to the figures below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q159: According to the periodic table, the compound
Q160: Although air temperatures at a site on
Q162: Which shows the elements carbon (C), hydrogen
Q163: Polar molecules<br>A) have electric charges that are
Q164: How does the ionic compound magnesium chloride
Q165: Ice floats because the ice crystals<br>A) contain
Q166: All of the elements listed below follow