Multiple Choice
Refer to the figures below. 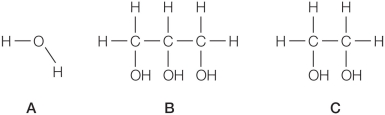 Rank the compounds in order of lowest to highest heat capacity per mole of compound.
Rank the compounds in order of lowest to highest heat capacity per mole of compound.
A) C < A < B
B) A < C < B
C) B < A < C
D) B < C < A
E) C < B < A
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q153: Which element has a higher atomic mass
Q154: Given that Avogadro's number is 6.02 ×
Q155: Which correctly ranks the relative strengths of
Q156: The mass number of an atom is
Q157: The covalent bonds in methane, CH<sub>4</sub>, are
Q159: According to the periodic table, the compound
Q160: Although air temperatures at a site on
Q161: Refer to the Bohr model of methane
Q162: Which shows the elements carbon (C), hydrogen
Q163: Polar molecules<br>A) have electric charges that are