Multiple Choice
Refer to the figure below. 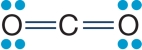 The figure shows the molecular structure of carbon dioxide.Carbon dioxide is nonpolar, whereas water is polar.Which of the true statements below explains these differences?
The figure shows the molecular structure of carbon dioxide.Carbon dioxide is nonpolar, whereas water is polar.Which of the true statements below explains these differences?
A) Carbon dioxide does not contain any polar covalent bonds, whereas water does.
B) Carbon dioxide contains only double bonds, whereas water contains only single bonds.
C) Carbon dioxide is a linear molecule, whereas water has a bent shape.
D) Carbon dioxide contains carbon atoms, whereas water does not.
E) Carbon and oxygen do not differ greatly in electronegativity, whereas hydrogen and oxygen do.
Correct Answer:

Verified
Correct Answer:
Verified
Q67: A neon atom has a total of
Q68: What is the molarity of 14.5 g
Q69: Refer to the figure below showing part
Q70: A mole of hydrogen and a mole
Q71: Phosphate ion has the structure PO<sub>4</sub><sup>3−</sup>.This ion
Q73: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q74: Which compound is expected to have a
Q75: A fish swimming in a shallow pool
Q76: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q77: The tendency of atoms in stable molecules