Multiple Choice
Refer to the table below. 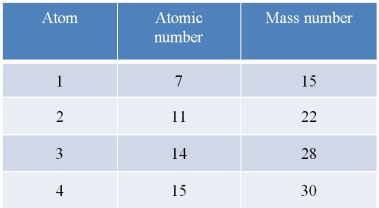 Which statement about the atoms in the table is accurate?
Which statement about the atoms in the table is accurate?
A) Atom 1 and atom 2 are isotopes of the same element.
B) Atom 1 and atom 4 have the same number of electrons in their outer shells.
C) Atom 3 and atom 4 have the same number of neutrons in their nuclei.
D) Atom 2 and atom 3 differ by six neutrons in their nuclei.
E) Atom 1 and atom 4 gain stability when they each lose one electron.
Correct Answer:

Verified
Correct Answer:
Verified
Q68: What is the molarity of 14.5 g
Q69: Refer to the figure below showing part
Q70: A mole of hydrogen and a mole
Q71: Phosphate ion has the structure PO<sub>4</sub><sup>3−</sup>.This ion
Q72: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q74: Which compound is expected to have a
Q75: A fish swimming in a shallow pool
Q76: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q77: The tendency of atoms in stable molecules
Q78: Which statement is false?<br>A) Covalent bonds can