Multiple Choice
Refer to the figure below. 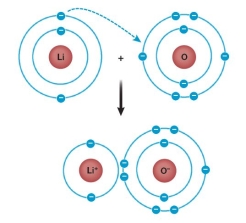 Does this figure accurately show how the ionic compound lithium oxide forms?
Does this figure accurately show how the ionic compound lithium oxide forms?
A) Yes, because it shows two atoms forming ions that attract one another.
B) Yes, because it shows how lithium and oxygen share electrons to form a bond.
C) No, because lithium should gain electrons from oxygen, not the other way around, as shown.
D) No, because oxygen has an incomplete outer shell and needs an electron from a second lithium atom.
E) No, because the figure does not show how lithium and oxygen share electrons.
Correct Answer:

Verified
Correct Answer:
Verified
Q122: Cholesterol is a lipid most often found
Q123: Which observation illustrates how water's heat of
Q124: The pH scale is a logarithmic scale.This
Q125: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q126: Which of the elements listed below requires
Q128: Refer to the balanced chemical equation below.<br>
Q129: Hydrogen bonds are attractions<br>A) between oppositely charged
Q130: Covalent bond formation depends on the ability
Q131: Oxygen, which has an electronegativity of 3.5,
Q132: Refer to the balanced chemical equation below.<br>2