Multiple Choice
Refer to the table below. 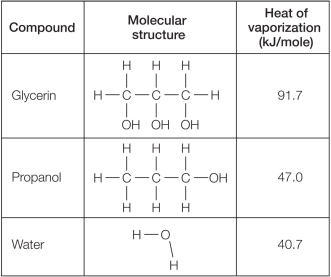 Which statement explains reasons for differences in the heat of vaporization for these compounds?
Which statement explains reasons for differences in the heat of vaporization for these compounds?
A) Glycerin's high heat of vaporization compared with that of the other molecules can be explained because it is both polar and capable of forming hydrogen bonds.
B) The heat of vaporization of glycerin is twice as high as that of water because glycerin is so much larger in size than water.
C) The greater hydrogen-bonding capacity of glycerin explains its greater heat of vaporization when compared with water or propanol.
D) Water has a lower heat of vaporization compared with glycerin and propanol because its hydrogen bonds are weaker.
E) Molecules that are polar and capable of forming hydrogen bonds tend to have similar heats of vaporization.
Correct Answer:

Verified
Correct Answer:
Verified
Q120: Which interaction between atoms is the strongest?<br>A)
Q121: All of the following are nonpolar except<br>A)
Q122: Cholesterol is a lipid most often found
Q123: Which observation illustrates how water's heat of
Q124: The pH scale is a logarithmic scale.This
Q126: Which of the elements listed below requires
Q127: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q128: Refer to the balanced chemical equation below.<br>
Q129: Hydrogen bonds are attractions<br>A) between oppositely charged
Q130: Covalent bond formation depends on the ability