Multiple Choice
The figure shows a PV diagram for 8.3 g of nitrogen gas in a sealed container.The temperature of state 1 is 79°C.What are (a) pressure p1 and (b) temperature T2? 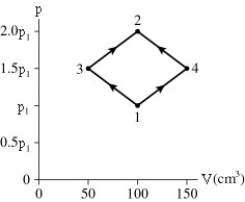
A) (a) 86 atm (b) 700°C
B) (a) 19 atm (b) 700°C
C) (a) 86 atm (b) 160°C
D) (a) 19 atm (b) 160°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: The diagrams shows a PV diagram for
Q61: The amount of heat needed to raise
Q62: The amount of heat needed to raise
Q63: Dust particles are pulverized rock,which has density
Q64: 52 g of methane (CH<sub>4</sub>)contain 3.25 moles
Q66: A 24.0 kg sample of ice is
Q67: A cylinder fitted with a movable piston
Q68: The temperature is increased from 20°C to
Q69: As you add heat to boiling water,its
Q70: The oxygen molecules and the nitrogen molecules