Multiple Choice
The diagrams shows a PV diagram for 4.3 g of oxygen gas in a sealed container.The temperature of state 1 is 21°C.What are the temperatures T3 and T4? 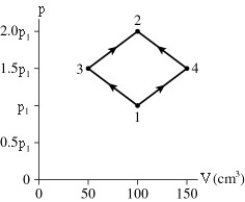
A) -52°C and 390°C
B) 16°C and 47°C
C) 220°C and 660°C
D) 11°C and 32°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: If you wanted to know how much
Q56: Some properties of glass are listed here.
Q57: In an isothermal process,the gas temperature always
Q58: 1.0 mol of an elemental solid and
Q59: A 960.0 g iron meteor impacts the
Q61: The amount of heat needed to raise
Q62: The amount of heat needed to raise
Q63: Dust particles are pulverized rock,which has density
Q64: 52 g of methane (CH<sub>4</sub>)contain 3.25 moles
Q65: The figure shows a PV diagram for