Multiple Choice
An electron inside a hydrogen atom is confined to within a space of 0.110 nm. What is the minimum uncertainty in the electron's velocity? ( 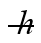 = 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
= 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
A) 5.26 × 105 m/s
B) 7.50 × 105 m/s
C) 5.26 × 107 m/s
D) 7.50 × 107 m/s
E) 5.26 × 109 m/s
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A laser emits light of wavelength 463
Q3: A molecule of roughly spherical shape has
Q4: A 440-nm spectral line is produced by
Q5: When a certain metal is illuminated by
Q6: An 84-kW AM radio station broadcasts at
Q7: X-rays of energy 2.9 × 10<sup>4</sup> eV
Q8: In a particular case of Compton scattering,
Q9: A metal surface has a work function
Q10: A measurement of an electron's speed is
Q11: A laser produces a beam of 4000-nm