Multiple Choice
Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values 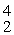 He: 4.002603 u
He: 4.002603 u 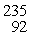
U: 235.043924 u
What is the mass of 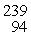
Pu in atomic mass units? (1 u = 931.494 MeV/c2)
A) 239.05215 u
B) 239.02775 u
C) 239.00189 u
D) 238.99919 u
E) 238.98884 u
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Calculate the amount of energy that is
Q4: A 57-kg researcher absorbs 6.3 × 10<sup>8</sup>
Q5: Two deuterium nuclei, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="Two deuterium
Q6: How many days are required for a
Q9: The decay rate of an isotope is
Q10: What would be the expected radius of
Q11: The primary source of the energy radiated
Q70: If a nucleus decays by β- decay
Q76: The reaction energy (Q value)for a particular
Q83: The half-life of cobalt-60 is 5.3 years,while