Multiple Choice
Two deuterium nuclei, 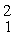 H, fuse to produce a tritium nucleus,
H, fuse to produce a tritium nucleus, 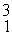
H, and a hydrogen nucleus. A neutral deuterium atom has a mass of 2.014102 u; a neutral tritium atom has a mass of 3.016049 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007276 u. How much energy is released in the process? (1 u = 931.494 MeV/c2)
A) 3.03 MeV
B) 3.53 MeV
C) 4.03 MeV
D) 4.53 MeV
E) 6.58 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q1: An ancient rock is found to contain
Q2: Calculate the amount of energy that is
Q4: A 57-kg researcher absorbs 6.3 × 10<sup>8</sup>
Q6: How many days are required for a
Q7: Plutonium-239 decays into uranium-235 plus an alpha
Q9: The decay rate of an isotope is
Q10: What would be the expected radius of
Q11: The primary source of the energy radiated
Q70: If a nucleus decays by β- decay
Q76: The reaction energy (Q value)for a particular