Multiple Choice
Scandium, 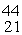 Sc, decays by emitting a positron. What is the nuclide that is the product of the decay?
Sc, decays by emitting a positron. What is the nuclide that is the product of the decay?
A)  Sc
Sc
B)  Sc
Sc
C)  Ca
Ca
D)  Ca
Ca
E)  Sc
Sc
Correct Answer:

Verified
Correct Answer:
Verified
Q8: The maximum permissible workday dose for occupational
Q65: A radioactive sample has a half-life of
Q71: What would be the expected radius of
Q72: Carbon-14 has a half-life of 5730 y.A
Q82: Consider the fusion reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="Consider
Q85: Uranium-238 decays into thorium-234 plus an alpha
Q86: In the fission reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="In
Q88: A proton strikes an <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="A
Q89: How does the mass of the products
Q89: A 70-kg laboratory technician absorbs 2.9 mJ