Short Answer
Consider the fusion reaction 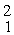
H + 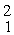
H + 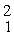
H → 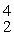
He + 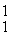
H + 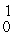
n
The atomic masses are: 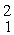
H: 2.01410 u 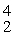
He: 4.00260 u 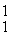
H, 1.00783 u 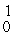
n: 1.008665 u
What mass of deuterium fuel, 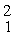
H, is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Correct Answer:

Verified
Correct Answer:
Verified
Q8: The maximum permissible workday dose for occupational
Q65: A radioactive sample has a half-life of
Q71: What would be the expected radius of
Q72: Carbon-14 has a half-life of 5730 y.A
Q77: How much energy is released in the
Q78: Two deuterium nuclei, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="Two deuterium
Q84: Scandium, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="Scandium, Sc,
Q85: Uranium-238 decays into thorium-234 plus an alpha
Q86: In the fission reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="In
Q89: How does the mass of the products